|
One property of matter is the electric
charge. Most sub-atomic particles have either a positive
(+) or negative (-) electrical charge. Those that don't
are considered neutral. The most common charged
particles are the electron and proton. Atoms with an
excess of electrons are called negative ions. Those with
missing electrons are called positive ions. There is an
electrical field that flows between opposite charges,
causing an electrical force. This results in an
attractive force between the opposite charges and a
repelling force between like charges.
Questions you may have are:
-
Which particles have charges?
-
What does the electric field look
like?
-
What does the electrical force do?
Charged particles
An atom is comprised of a nucleus
consisting of protons and neutrons and a collection of
electrons in orbits or shells around the nucleus.
Protons, neutrons and electrons are the most common
sub-atomic particles. (See Sub-Atomic Particles for more
information.)
Sub-atomic particles have a positive (+)
electrical charge, a negative (-) electrical charge, or
no electrical charge at all. For example, a proton has a
positive electric charge, an electron has a negative
electric charge, and a neutron is neutral and has no
electrical charge.
An atom typically has the same number of
negative charged electrons as positive charged protons,
so its total charge is neutral. But if the atom loses
some electrons, it will have more positive charges than
negative charges and is called a positive ion. Likewise,
if the atom gains an excess of electrons, it is called a
negative ion.
Ions are charged particles. They are
often involved in static electricity and electrical
current on electrolyte solutions such as salt water.
Electrically charged particles are
called unipoles, in that they can exist by themselves ("uni"
means one). This is different than the case of magnetic
poles, where for every N pole, there must be an S pole.
Magnets are called dipoles, meaning they must have two
poles.
There is duality in the Universe. That
means if there is a left hand, there will be a right
hand. That also works with particles.
Since there is a negative charged
electron, there is also a version with a positive
charge. That anti-electron particle is called the
positron. It is the same size and weight as an electron,
except it has an opposite charge.
For the positive charged proton, there
is the anti-proton that has a negative charge. These
oppositely charged particles are called anti-matter.
There is even an anti-neutron. It is
still neutral in electrical charge, but it spins in the
opposite direction.
Electrical field
An electrical field surrounds every
particle that has an electrical charge. By convention,
the lines of the electric field are said to radiate from
a (+) particle and move towards a (-) particle. It is
not certain if there is any direction of radiation, and
there is no real good explanation of what the electric
field is made of. It's just there.

Electric field lines shown moving from a
positive particle
When a positive charged particle (+)
like a proton is near a negative charged particle (-)
like an electron, the electrical field goes from one to
the other.
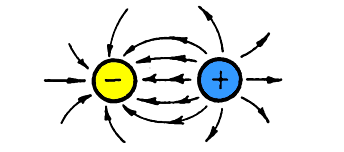
Electrical field points from (+) to (-)
Forces acting on charged particles
The electrical field acts like a force
at a distance and the lines are considered lines of
force.
When a positive charged particle is near
a negative charged particle, they are attracted to each
other by the lines of force.
Static electricity is a good example of
opposite charges attracting. If electrons are collected
on the surface of one material and positive ions are
collected on another surface, the negative and positive
charges attract. Either the materials are pulled
together or a stream of electrons jumps the gap as a
spark.
Note that since protons are in the
nucleus, they never collect on a surface in static
electricity. Rather, they contribute to the charge of
the ions that have lost electrons.
If an electron would come near its
anti-matter twin, the positron, they would be attracted
to each other until they collide. Then they would each
be annihilated with a large amount of energy given off
in the form of electromagnetic radiation and other
smaller sub-atomic particles moving at a high speed.
Likewise, if a proton comes into contact with an
anti-proton, they will annihilate, giving off a large
amount of energy.
Although they have opposite charges and
are attracted to each other, an electron will never
combine with a proton. The reason has to do with other
forces being involved that keep them apart, as explained
in Quantum Theory. This is the same reason that
electrons don't go crashing into the positive charged
atomic nucleus.
When particles have the same charge,
they repel each other.
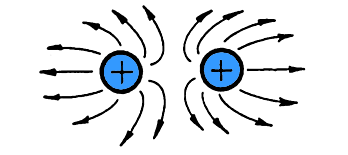
Like charges push away from each other
This can be seen in a static electricity
experiment. Attach strings to two balloons and rub them
both on a wool sweater. Then when you hang the balloons
next to each other, you can see the electrical forces
push them apart.
An important property of matter is
electric charge. Most sub-atomic particles have either a
positive (+) or negative (-) electrical charge. Those
that don't are considered neutral. Atoms with an excess
of electrons or are missing electrons are called ions.
There is an electrical field that flows between the
positive to negative charges, resulting in an attractive
force between the two. Opposite charges attract and like
charges repel. |