|
Moving or spinning electrical charges
such as electrons create a magnetic field with N and S
poles. If most of the poles are aligned in the same
direction, the material is magnetic. The factors that
determine the way a material responds to a magnetic
field are the alignment of electrons in the atom, of the
atoms in a molecule or alloy, and the domains within the
material.
Since the atoms or molecules need to be
aligned, gases and liquids are typically not magnetic,
because of the free motion of the particles. There are
some exceptions, especially concerning the plasma state
of matter. Typically, all magnets are solid metals.
Questions you may have include:
Moving electrons create magnetic field
When electrons move, they create a
magnetic field. Placing a compass near a wire carrying
DC electrical current can show that a magnetic field is
created. The field is also created when electrons rotate
around a nucleus and when they spin while in orbit.
Electrons have a property called spin.
This spinning creates a magnetic field with N and S
poles, just as the spinning Earth has magnetic poles.
Note that the N pole on an electron is really a
North-seeking pole, just as in a magnet.
If electrons in the shells of an atom
spin in the same direction, the atom will exhibit a
magnetic field and will respond to the forces of a
magnet. If half of the electrons spin one way and the
rest spin the other way, they will neutralize each other
and the material will not be affected by a magnetic
field
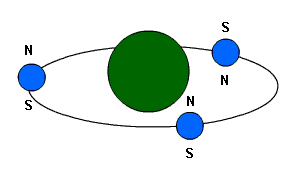
This atom is barely magnetic because all
its electrons are not aligned
Atoms such as iron have most of their
electrons aligned in the same direction. Thus, iron or
nickel would be attracted to a magnet. An element with
half of its electrons oriented one way would not be
attracted to a magnet.
In the Bohr--or solar system--model of
an atom, the electrons orbit around the nucleus. This
motion also creates a magnetic field. The atom will have
N and S poles, but the orientation of the electrons will
determine whether the atom has a strong magnetic field
or weak field.
Molecular alignment
If atoms having a strong magnetic field
are aligned within a molecule, it too will be magnetic.
If atoms are facing different directions, their fields
will cancel out each other.
Although some atoms may be highly
magnetic, they really need to be aligned to make a
material magnetic. If two or more elements are
chemically combined to form a molecule, it is quite
possible that the compound is not very magnetic, because
the orientations of the atoms in the molecule work
against each other. A good example of this is to compare
the magnetic properties of iron as compared to its
compounds if iron oxide (rust) and iron sulfide.
Metals of different elements can be
mixed when they are in the molten or liquid state to
form alloys. These combinations result in materials with
slightly different physical and chemical properties than
the elements by themselves.
If the metals typically respond well to
a magnetic field--such as iron and nickel--then their
alloy has even a stronger reaction to magnetism. On the
other hand, there are some alloys of iron--such as forms
of stainless steel--that do not respond well at all to a
magnet.
The final factor in a material being
magnetic concern the orientation of its domains. A group
of atoms in a metal may become aligned, but the various
groups may be misaligned. These groups are called
domains.
It is necessary to line up many of the
domains in a material like iron in order for it to
become a magnet.
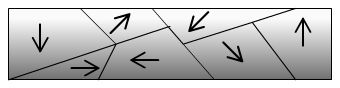
Magnetic material with domains
misaligned
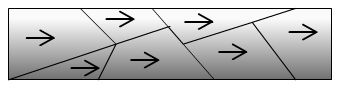
Aligned domains makes material highly
magnetic
Alignment of electrons, atoms, and
domains are important in determining the magnetic
response of a material and whether it is a magnet. Since
the atoms or molecules need to be aligned, gases and
liquids are typically not magnetic, and most magnets are
solid metals. |