Conductors,
insulators, and electron flow
The electrons of different types of atoms
have different degrees of freedom to move around. With some
types of materials, such as metals, the outermost electrons
in the atoms are so loosely bound that they chaotically move
in the space between the atoms of that material by nothing
more than the influence of room-temperature heat energy.
Because these virtually unbound electrons are free to leave
their respective atoms and float around in the space between
adjacent atoms, they are often called free electrons.
In other types of materials such as glass,
the atoms' electrons have very little freedom to move
around. While external forces such as physical rubbing can
force some of these electrons to leave their respective
atoms and transfer to the atoms of another material, they do
not move between atoms within that material very easily.
This relative mobility of electrons within a
material is known as electric conductivity.
Conductivity is determined by the types of atoms in a
material (the number of protons in each atom's nucleus,
determining its chemical identity) and how the atoms are
linked together with one another. Materials with high
electron mobility (many free electrons) are called
conductors, while materials with low electron mobility
(few or no free electrons) are called insulators.
Here are a few common examples of conductors
and insulators:
-
Conductors:
-
silver
-
copper
-
gold
-
aluminum
-
iron
-
steel
-
brass
-
bronze
-
mercury
-
graphite
-
dirty water
-
concrete
-
Insulators:
-
glass
-
rubber
-
oil
-
asphalt
-
fiberglass
-
porcelain
-
ceramic
-
quartz
-
(dry) cotton
-
(dry) paper
-
(dry) wood
-
plastic
-
air
-
diamond
-
pure water
It must be understood that not all
conductive materials have the same level of conductivity,
and not all insulators are equally resistant to electron
motion. Electrical conductivity is analogous to the
transparency of certain materials to light: materials that
easily "conduct" light are called "transparent," while those
that don't are called "opaque." However, not all transparent
materials are equally conductive to light. Window glass is
better than most plastics, and certainly better than "clear"
fiberglass. So it is with electrical conductors, some being
better than others.
For instance, silver is the best conductor
in the "conductors" list, offering easier passage for
electrons than any other material cited. Dirty water and
concrete are also listed as conductors, but these materials
are substantially less conductive than any metal.
Physical dimension also impacts
conductivity. For instance, if we take two strips of the
same conductive material -- one thin and the other thick --
the thick strip will prove to be a better conductor than the
thin for the same length. If we take another pair of strips
-- this time both with the same thickness but one shorter
than the other -- the shorter one will offer easier passage
to electrons than the long one. This is analogous to water
flow in a pipe: a fat pipe offers easier passage than a
skinny pipe, and a short pipe is easier for water to move
through than a long pipe, all other dimensions being equal.
It should also be understood that some
materials experience changes in their electrical properties
under different conditions. Glass, for instance, is a very
good insulator at room temperature, but becomes a conductor
when heated to a very high temperature. Gases such as air,
normally insulating materials, also become conductive if
heated to very high temperatures. Most metals become poorer
conductors when heated, and better conductors when cooled.
Many conductive materials become perfectly conductive (this
is called superconductivity) at extremely low
temperatures.
While the normal motion of "free" electrons
in a conductor is random, with no particular direction or
speed, electrons can be influenced to move in a coordinated
fashion through a conductive material. This uniform motion
of electrons is what we call electricity, or
electric current. To be more precise, it could be called
dynamic electricity in contrast to static
electricity, which is an unmoving accumulation of electric
charge. Just like water flowing through the emptiness of a
pipe, electrons are able to move within the empty space
within and between the atoms of a conductor. The conductor
may appear to be solid to our eyes, but any material
composed of atoms is mostly empty space! The liquid-flow
analogy is so fitting that the motion of electrons through a
conductor is often referred to as a "flow."
A noteworthy observation may be made here.
As each electron moves uniformly through a conductor, it
pushes on the one ahead of it, such that all the electrons
move together as a group. The starting and stopping of
electron flow through the length of a conductive path is
virtually instantaneous from one end of a conductor to the
other, even though the motion of each electron may be very
slow. An approximate analogy is that of a tube filled
end-to-end with marbles:
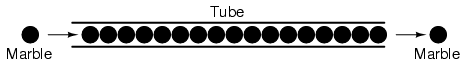
The tube is full of marbles, just as a
conductor is full of free electrons ready to be moved by an
outside influence. If a single marble is suddenly inserted
into this full tube on the left-hand side, another marble
will immediately try to exit the tube on the right. Even
though each marble only traveled a short distance, the
transfer of motion through the tube is virtually
instantaneous from the left end to the right end, no matter
how long the tube is. With electricity, the overall effect
from one end of a conductor to the other happens at the
speed of light: a swift 186,000 miles per second!!! Each
individual electron, though, travels through the conductor
at a much slower pace.
If we want electrons to flow in a certain
direction to a certain place, we must provide the proper
path for them to move, just as a plumber must install piping
to get water to flow where he or she wants it to flow. To
facilitate this, wires are made of highly conductive
metals such as copper or aluminum in a wide variety of
sizes.
Remember that electrons can flow only when
they have the opportunity to move in the space between the
atoms of a material. This means that there can be electric
current only where there exists a continuous path of
conductive material providing a conduit for electrons to
travel through. In the marble analogy, marbles can flow into
the left-hand side of the tube (and, consequently, through
the tube) if and only if the tube is open on the right-hand
side for marbles to flow out. If the tube is blocked on the
right-hand side, the marbles will just "pile up" inside the
tube, and marble "flow" will not occur. The same holds true
for electric current: the continuous flow of electrons
requires there be an unbroken path to permit that flow.
Let's look at a diagram to illustrate how this works:

A thin, solid line (as shown above) is the
conventional symbol for a continuous piece of wire. Since
the wire is made of a conductive material, such as copper,
its constituent atoms have many free electrons which can
easily move through the wire. However, there will never be a
continuous or uniform flow of electrons within this wire
unless they have a place to come from and a place to go.
Let's add an hypothetical electron "Source" and
"Destination:"

Now, with the Electron Source pushing new
electrons into the wire on the left-hand side, electron flow
through the wire can occur (as indicated by the arrows
pointing from left to right). However, the flow will be
interrupted if the conductive path formed by the wire is
broken:
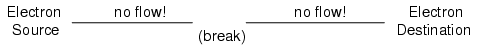
Since air is an insulating material, and an
air gap separates the two pieces of wire, the
once-continuous path has now been broken, and electrons
cannot flow from Source to Destination. This is like cutting
a water pipe in two and capping off the broken ends of the
pipe: water can't flow if there's no exit out of the pipe.
In electrical terms, we had a condition of electrical
continuity when the wire was in one piece, and now that
continuity is broken with the wire cut and separated.
If we were to take another piece of wire
leading to the Destination and simply make physical contact
with the wire leading to the Source, we would once again
have a continuous path for electrons to flow. The two dots
in the diagram indicate physical (metal-to-metal) contact
between the wire pieces:
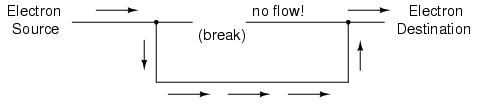
Now, we have continuity from the Source, to
the newly-made connection, down, to the right, and up to the
Destination. This is analogous to putting a "tee" fitting in
one of the capped-off pipes and directing water through a
new segment of pipe to its destination. Please take note
that the broken segment of wire on the right hand side has
no electrons flowing through it, because it is no longer
part of a complete path from Source to Destination.
It is interesting to note that no "wear"
occurs within wires due to this electric current, unlike
water-carrying pipes which are eventually corroded and worn
by prolonged flows. Electrons do encounter some degree of
friction as they move, however, and this friction can
generate heat in a conductor. This is a topic we'll explore
in much greater detail later.
-
REVIEW:
-
In conductive materials, the outer
electrons in each atom can easily come or go, and are
called free electrons.
-
In insulating materials, the outer
electrons are not so free to move.
-
All metals are electrically conductive.
-
Dynamic electricity, or electric
current, is the uniform motion of electrons through a
conductor. Static electricity is an unmoving,
accumulated charge formed by either an excess or
deficiency of electrons in an object.
-
For electrons to flow continuously
(indefinitely) through a conductor, there must be a
complete, unbroken path for them to move both into and out
of that conductor.
|